-
Propietary Products
Propietary Products
Our speciality is the development and manufacture of precision parts and assemblies with break-off, tear-off or twist-off zones in all injection-moldable materials.
- Solutions
Solutions
A question of perspective:
You might think we can't do much ... just plastic parts for use in the medical industy.
On the other hand, we can do nothing better than that: we concentrate on our core competencies.
Without compromise - best quality - at a reasonable price - Made in Germany
Together with our customers, we develop precision parts, assemblies and ready-to-use packaged complete systems from the idea to series production.- About us
- Quality Management
- Career
- News
- Contact
Clean room Assembly
We know our responsibility for products that later serve the health of humans and animals.
Clean room Assembly
Enormous demands are placed on medical products in terms of hygiene and cleanliness.
We know our responsibility for products that later serve the health of humans and animals.
And we have more than 30 years of experience in cleanroom production. Regular particle and germ measurements ensure purity.
The entire production chain can be offered from laminar flow to ISO Class 8 cleanroom conditions, depending on requirements.
We have high manufacturing flexibility - from prototype to series production.
On request, even large quantities can be produced, processed and assembled under cleanroom conditions.
So we know what we're talking about - and offer flexible solutions, whether in production, assembly or packaging.
- Monitored and controlled cleanroom conditions - according to ISO Class 8
- Laminar flow purity up to class 10 000
- Cleanroom production with 4 injection moulding machines
- 200m² production area (RR21)
- 3-shift system
- Ready-to-use products
- Complex electronic semi-assemblies
- Automatic assembly with camera monitoring
- Manual assembly of complex subassemblies
- Adhesive bonding of plastics
- Various sterile packaging processes (welding + sealing semi-automated, ultrasonic welding)
- integrated testing processes (see Palamix vacuum and Oculus distortion)
- Trained personnel for monitoring product and hygiene
- Partial 100% inspection and testing
- Weighing - labelling - packaging
Contact
Tel.: +49 6172 9570-0
E-mail: info@spang-brands.de
- Solutions
 2port caps
2port caps
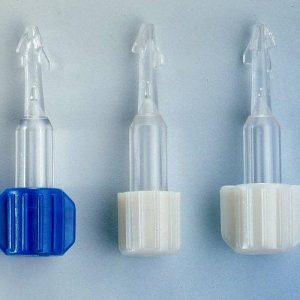 Breakable caps, assembled
Breakable caps, assembled
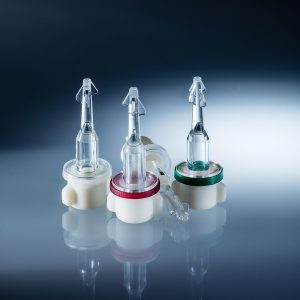 Tamper Proof Caps
Tamper Proof Caps
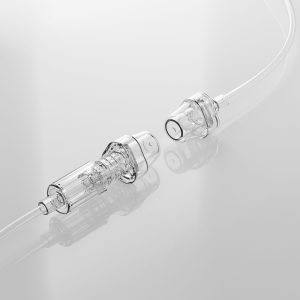 Steril Connector
Steril Connector
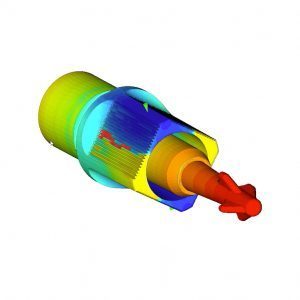 Product Development
Product Development
 Mold Shop
Mold Shop
 Injection Moulding
Injection Moulding
 Clean room Assembly
Clean room Assembly